
MEASURING & USING SPECIFIC GRAVITIES OF LEAD ALLOYS
Retrieved: 01/08/2015
Posted 07/27/2014
The subject article is posted with permission from SPG, Inc. It was published in the Summer 2014 edition (Issue #86) of the Black Powder Cartridge magazine.
But before getting into the article and as a reference, listed below are the SG values of several well known bullet casting alloys. If you don't have access to an alloy calculator such as the one referenced in the article, once the SG is measured you should be able to make a rough estimate of the lead/tin percentage from the following values.
SG of pure lead is 11.3450
SG of 30:1 (lead/tin) is 11.1485
SG of 25:1 (lead/tin) is 11.1115
SG of 20:1 (lead/tin) is 11.0574
SG of 16:1 (lead/tin) is 10.9918
SG of pure tin is 7.337
Note - If there are elements in the alloy other than lead or tin the above SGs do not apply. Therefore the values cannot be used for wheelweight alloys that contain antimony, arsenic or other stuff. For alloys containing lead, tin & antimony here's a link to an article containing an excellent chart of SGs:
Determining Alloy composition.pdf (at castpics.net)
Determining Alloy
composition.pdf (local copy)
By the way, although I used a digital scale for the article, with a little ingenuity a beam balance scale will also work
****************************************************************
You may be wondering what lead alloys and Specific Gravity have in common and expecting you to remember back to your high school or college general physics or chemistry class is probably a stretch, especially for us older "codgers." So allow me to refresh your memory. Specific Gravity (SG), also known as Relative Density, is the ratio of the density of any substance to the density of a standard substance, water being the standard for liquids and solids. To be precise, the SG of a solid or liquid is usually measured at a temperature of 20degC and compared to the density of distilled water at 4degC. Therefore a substance with a SG less than 1 will float on water, and will sink if the SG is greater than 1. We have the Greek mathematician Archimedes to thank for discovering SG around 212 B.C. So what does SG has to do with lead alloys and bullet casting?
In many cases, using SG, the identity of an unknown element or simple alloy components can be determined. One example is alloys of lead and tin. I was reintroduced to SG recently after posting on a well known BPCR forum that a .40 caliber bullet cast from 20:1 (lead/tin) alloy weighed 420 grains and the same bullet weighed 427 grains when cast from 25:1 alloy. Reading my post, an astute shooter and experimenter with an engineering background took exception to the alloy mix versus bullet weights and provided a convincing argument, using a simple SG formula, that the increase in weight should be much smaller, only 1.35 grains to be precise.
For some time I'd considered purchasing or making a SG measuring setup to confirm the purity of the lead and tin purchased and the mix percentage of lead/tin alloys. Until recently all my bullet alloys were mixed from reportedly pure lead and tin, therefore a tester was not essential. Now the contents of two alloys were in question and SGs were required in order to determine the actual lead/tin concentrations. Having mixed one batch from pure lead and tin I was confident it was close to 20:1. The other batch, reported to be 25:1 but now in question, was from a friend. To determine the actual mix a SG measuring setup was needed.

SG Measuring Techniques
Archimedes' principal states that "Any object, wholly or partially immersed in a fluid, is buoyed up by a force equal to the weight of the fluid displaced by the object." Therefore, depending on the measurement technique detailed here, two formulas are applicable. Formula A: SG = weight of object in air ÷ weight of object in water. Formula B: SG = weight of object in air ÷ (weight of object in air - weight of object in water). To simplify the formulas the weight of the object in air will be referred to as the “dry weight” and the weight of the object in water will be the “wet weight”. Hence, to obtain the necessary values for the calculations, the object is 1st weighed in air then weighed again while suspended in water, referred to as hydrostatic weighing. In theory this seemed easy enough but a digital scale is required. Ideally the scale should be capable of a resolution of 0.01 grains, but 0.1 grains is sufficient if a careful measurement technique is used as explained later.
SG measuring setups are available from numerous laboratory equipment manufacturers. An example of one setup is shown in Figure 1. But even simpler solutions will suffice for the bullet caster. After additional research I realized the platform shown in Figure 1 to hold the cup of water is not absolutely necessary. In fact a reasonably accurate measurement can be made without the wire frame fixture to hold the bullet or object.

Very Simple SG Measurement Technique
The simplest procedure uses a small container of water placed directly on the scale platen. The container should be as lightweight as possible but large enough to hold a submerged bullet without the bullet coming in contact with the container. I used a prescription pill container. Figure 2 illustrates the 3-step measurement procedure.
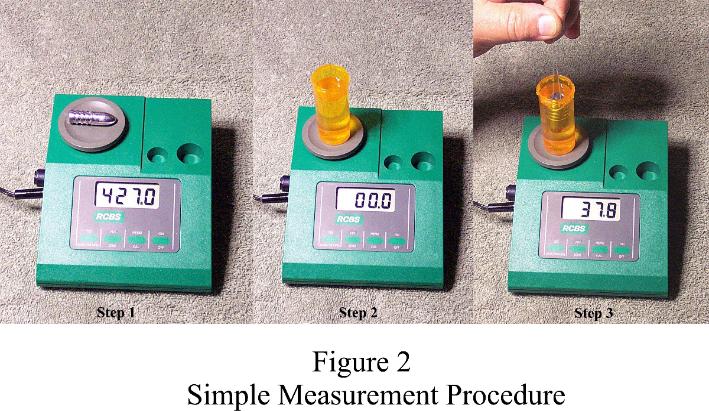
1. Assuming the scale is warmed up and calibrated, place the bullet on the platen. Measure and record the dry weight.
2. Place the container of water on the platen and zero out the tare weight. Note - If the scale does not have a zeroing or tare weight feature, the measurements can still be made but the scale must be capable of accurately measuring the total weight of the container of water and bullet to an accuracy of 0.1 grains minimum. In this case the weight of the container of water must be measured and subtracted from the wet measurements.
3. Using sewing thread or thin monofilament line, suspend the bullet fully submerged. Note the "wet weight" reading while ensuring the bullet does not contact the inside of the container.
4. Using the dry and wet weights, determine the SG of the bullet alloy. Formula A applies in this case. Using a dry weight of 427 grains and a wet weight of 37.8 grains, SG = 427 divided by 37.8 = 11.2963; rounded to 11.30. Note - Although the test sample and faucet water will be at room temperature, the resulting accuracy of the procedure will be sufficient for our needs assuming the scale has a resolution of at least 0.1 grains, several careful measurements are made and the results averaged. Out of curiosity I did try very cold distilled water and there was no measureable difference within the resolution of the RCBS scale.
Constructing a Test Fixture
Although the very simple procedure seemed to work fine, I wondered if the wire fixture on the commercial setup shown in Figure 1 offered an advantage. Using it as a model, the fixture displayed in Figure 3 was constructed. It had to be sufficiently lightweight to allow zeroing out its "tare weight." The fixture consists of a small cork, four pieces of thin wire, a thin plastic disk from a compact disk container and a lightweight plastic cap off an aerosol can. The cap is slightly larger in diameter than the scale platen. It was cut down to the thickness of the top of the platen and glued to the disk. Although not absolutely necessary, the cap centers the fixture and keeps it from sliding off the platen. The fixture is tall enough (8") to insert a small handheld cup of water under the bullet without spilling the water or touching anything. The space between the main support wires is sufficient to allow entry of my hands while holding the cup. The fixture weighs a little less than 400 grains.
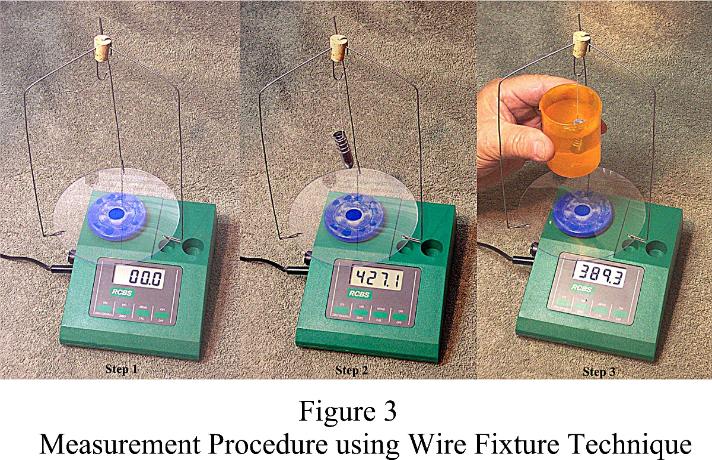
Technique Using the Wire Fixture to Measure SG<{> 1. Assuming the scale is warmed up and calibrated, install the fixture and zeroing out the tare weight.
2. Suspend the bullet on the hook using sewing thread or thin monofilament line. If everything is working correctly; the bullet should weigh the same as when it's placed directly on the scale platen.
3. The bullet is then suspended in water by simply raising the cup of water under the bullet until it's fully submerged. Make a mental note of the "wet weight" reading while holding the cup steady and ensuring the bullet does not contact the inside of the cup and nothing touches the fixture.
4. Using the dry and wet weights, determine the SG of the bullet alloy. Formula B applies for this procedure. Using a dry weight of 427 grains and a wet weight of 389.3 grains, SG = 427.1 divided by (427.1 minus 389.3) = 427.1 divided by 37.8 = 11.2989; rounded to 11.30.
Conclusion
Identical results were achieved by either technique. No doubt measuring the SG of very small or lightweight objects with a high resolution scale would benefit from the increased stability when using the wire fixture and water cup platform, but there's no obvious advantage to using the technique for bullet casting and lead alloy applications. Therefore the simpler procedure is the preferred technique.
Using SGs to Derive Alloy Weights & Mix
Using bullets from the two batches of alloy, 10 measurements were made of each batch. After tossing out the high and low values the remaining 8 were averaged.
The alloy I had mixed turned out to have a SG of 11.09, which equates to 22.8:1 with 4.2% tin, slightly off the goal of 20:1. The alloy that was reported to be 25:1 had a SG of 11.30, equating to a lead/tin ratio of 136:1 and containing only 0.73% tin. Hence, my friends alloy was way off the mark of 25:1. Using the correct SG values and 427 grains for the 136:1 alloy bullet, the 22.8:1 alloy should cast a bullet of 427 (11.09 divided by 11.30) = 419.06 grains; close enough for government work to the 420 grains I had posted on the forum.
Note - There is an excellent software-based casting alloy calculator available that I highly recommend. It offers several features including the ability to compute the percentage of lead and tin in the alloy based on SGs. For more details on the calculator go to http://tmtpages.com/Alloy/alloycalc.htm.
I used it to quickly derive the lead/tin ratios from the measured SGs.
For another example, let's determine what a bullet will weigh if the same mould is used but the alloy is changed. So what will a 545 grain bullet cast from 20:1 alloy weigh if cast with 30:1 alloy? First calculate the SG of the alloys using 11.345 for the SG of lead and 7.337 for the SG of Tin. By the way, it's a common misconception that SGs of an alloy can be accurately calculated by multiplying the weight or units of the alloy elements by their densities or SGs; add the results and divide by the total weight or units. The correct formula is more complex and is based on the reciprocals of the weights or units and densities or SGs. For example:
SG of 20:1 = 1 div [20 div (21 x 11.345) + 1 div (21 x 7.337)] = 11.0574
SG of 30:1 = 1 div [30 div (31 x 11.345) + 1 div (31 x 7.337)] = 11.1485
Notes - Depending on the source, published SGs of elements and alloys will vary slightly but will be well within the accuracy required for bullet casting. And when solving the formulas above, remember to follow the mathematical "Order of Operations" hierarchy, i.e. do things in parentheses first, multiply or divide before adding or subtracting and always go from left to right.
Knowing that a bullet cast with 30:1 will weigh more than one cast with 20:1, the next step is to multiply the bullet weight by the correct ratio of the SGs.
Therefore 545 (11.1485 ÷ 11.0574) = 549.49. Hence a 545 grain 20:1 alloy bullet will weigh approximately 549.5 grains if cast with 30:1 alloy from the same mould and under the same casting conditions.
Describing Lead/tin Alloys
Prior to closing this tutorial, I should mention there are several formats used to describe lead/tin alloys. Some shooters use all the formats interchangeably which is incorrect. Depending on the format used, there is a difference in the alloy mix although it's not large. The following examples should help clarify the differences.
20/1, 20-1, 1/20, 1-20 or 1 in 20 implies 95.0% lead, 5.0% tin
19 units of lead + 1 unit of tin = 20 units of alloy
9.5 units of lead + 0.5 units of tin = 10 units of alloy
30/1, 30-1, 1/30, 1-30 or 1 in 30 implies 96.67% lead, 3.33% tin
29 units of lead + 1 unit of tin = 30 units of alloy
14.5 units of lead + 0.5 units of tin = 15 units of alloy
1:30, 30:1 or 30 to 1 implies 96.77% lead, 3.23% tin
30 units of lead + 1 unit of tin = 31 units of alloy
15 units of lead + 0.5 units of tin = 15.5 units of alloy
1:40, 40:1 or 40 to 1 implies 97.56% lead, 2.44% tin
40 units of lead + 1 unit of tin = 41 units of alloy
20 units of lead + 0.5 units of tin = 20.5 units of alloy
Wishing you great shooting,
Wayne